Is Cellular Respiration an Anabolic or Catabolic Pathway? How Do You Know?
6.4: Cellular Respiration
- Page ID
- 35691
What you'll learn to do: Identify the reactants and products of cellular respiration and where these reactions occur in a jail cell

Almost every task performed by living organisms requires free energy. Energy is needed to perform heavy labor and exercise, just humans also apply free energy while thinking, and even during sleep. In fact, the living cells of every organism constantly use energy.
Nutrients and other molecules are imported into the cell, metabolized (cleaved downwardly) and perhaps synthesized into new molecules, modified if needed, transported around the cell, and possibly distributed to the entire organism. For example, the large proteins that make upward muscles are built from smaller molecules imported from dietary amino acids. Complex carbohydrates are broken down into simple sugars that the jail cell uses for energy.
Just equally energy is required to both build and demolish a edifice, free energy is required for the synthesis and breakdown of molecules every bit well as the send of molecules into and out of cells. In addition, processes such as ingesting and breaking downward pathogenic bacteria and viruses, exporting wastes and toxins, and movement of the cell require energy. From where, and in what class, does this energy come? How do living cells obtain energy, and how practice they employ it? This chapter volition discuss dissimilar forms of energy and the physical laws that govern energy transfer. This chapter will also describe how cells employ energy and replenish it, and how chemical reactions in the prison cell are performed with great efficiency.
In the process of photosynthesis, plants and other photosynthetic producers create glucose, which stores energy in its chemical bonds. Y'all will actually study photosynthesis in more than detail a bit later. But one time photosynthesis has created glucose to store free energy, both plants and consumers, such as animals, undergo a series of metabolic pathways, collectively called cellular respiration, to use that energy. Cellular respiration extracts the energy from the bonds in glucose and converts it into a form that all living things tin can use. Now permit's accept a more than detailed wait at how all eukaryotes—which includes humans!—brand use of this stored energy.
Learning Objectives
- Depict the process of glycolysis and identify its reactants and products
- Depict the procedure of the citric acid bike (Krebs cycle) and identify its reactants and products
- Describe the overall outcome of the citric acid wheel and oxidative phosphorylation in terms of the products of each
- Describe the location of the citric acid cycle and oxidative phosphorylation in the cell
Glycolysis
Fifty-fifty exergonic, energy-releasing reactions require a small corporeality of activation energy to proceed. However, consider endergonic reactions, which crave much more energy input because their products have more gratuitous energy than their reactants. Inside the cell, where does energy to power such reactions come from? The reply lies with an energy-supplying molecule chosen adenosine triphosphate, or ATP. ATP is a small, relatively simple molecule, but within its bonds contains the potential for a quick outburst of energy that tin exist harnessed to perform cellular piece of work. This molecule tin can be idea of as the primary energy currency of cells in the aforementioned way that money is the currency that people exchange for things they need. ATP is used to power the majority of energy-requiring cellular reactions.
ATP in Living Systems
A living jail cell cannot store meaning amounts of free energy. Excess energy would effect in an increment of oestrus in the prison cell, which would denature enzymes and other proteins, and thus destroy the cell. Rather, a cell must be able to store energy safely and release it for use but as needed. Living cells accomplish this using ATP, which can be used to fill whatsoever energy need of the cell. How? It functions as a rechargeable battery.
When ATP is broken downward, usually by the removal of its terminal phosphate grouping, energy is released. This energy is used to do work by the cell, usually by the binding of the released phosphate to some other molecule, thus activating information technology. For instance, in the mechanical work of muscle wrinkle, ATP supplies energy to motility the contractile muscle proteins.
ATP Construction and Part

At the center of ATP is a molecule of adenosine monophosphate (AMP), which is composed of an adenine molecule bonded to both a ribose molecule and a single phosphate group (Figure 2). Ribose is a v-carbon sugar found in RNA and AMP is one of the nucleotides in RNA. The addition of a 2d phosphate group to this core molecule results in adenosine diphosphate (ADP); the addition of a third phosphate grouping forms adenosine triphosphate (ATP).
The addition of a phosphate group to a molecule requires a high corporeality of energy and results in a high-free energy bail. Phosphate groups are negatively charged and thus repel one some other when they are arranged in series, as they are in ADP and ATP. This repulsion makes the ADP and ATP molecules inherently unstable. The release of ane or two phosphate groups from ATP, a procedure called hydrolysis, releases free energy.
Glycolysis
You lot have read that nearly all of the energy used by living things comes to them in the bonds of the saccharide, glucose. Glycolysis is the first stride in the breakdown of glucose to extract energy for prison cell metabolism. Many living organisms carry out glycolysis as part of their metabolism. Glycolysis takes place in the cytoplasm of most prokaryotic and all eukaryotic cells.
Glycolysis begins with the six-carbon, band-shaped structure of a unmarried glucose molecule and ends with two molecules of a three-carbon carbohydrate called pyruvate. Glycolysis consists of two singled-out phases. In the start part of the glycolysis pathway, energy is used to make adjustments so that the half dozen-carbon sugar molecule tin be divide evenly into ii 3-carbon pyruvate molecules. In the second part of glycolysis, ATP and nicotinamide-adenine dinucleotide (NADH) are produced (Effigy iii).
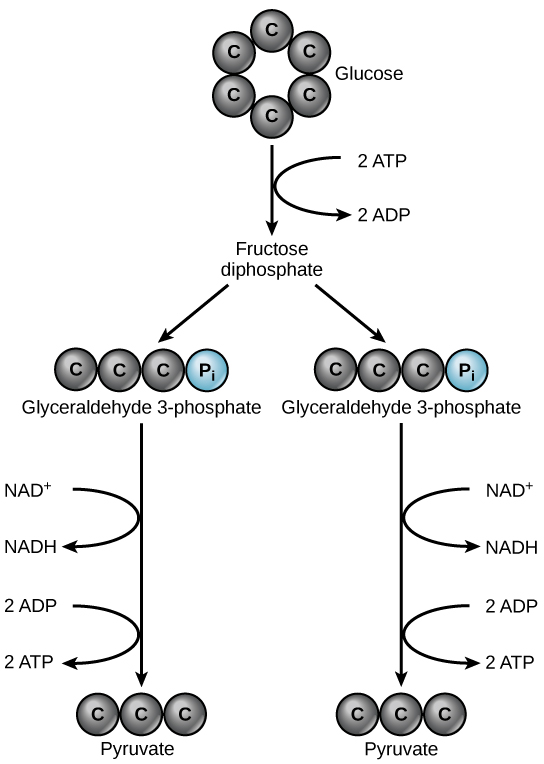
If the prison cell cannot catabolize the pyruvate molecules further, it will harvest only two ATP molecules from ane molecule of glucose. For example, mature mammalian crimson claret cells are only capable of glycolysis, which is their sole source of ATP. If glycolysis is interrupted, these cells would eventually die.
Learning Objectives
ATP functions equally the energy currency for cells. It allows cells to store energy briefly and transport it within itself to support endergonic chemic reactions. The structure of ATP is that of an RNA nucleotide with three phosphate groups attached. As ATP is used for free energy, a phosphate group is detached, and ADP is produced. Energy derived from glucose catabolism is used to recharge ADP into ATP.
Glycolysis is the beginning pathway used in the breakdown of glucose to extract free energy. Because it is used by most all organisms on world, information technology must take evolved early in the history of life. Glycolysis consists of two parts: The start part prepares the vi-carbon ring of glucose for separation into two iii-carbon sugars. Energy from ATP is invested into the molecule during this step to energize the separation. The second half of glycolysis extracts ATP and high-free energy electrons from hydrogen atoms and attaches them to NAD+. Two ATP molecules are invested in the first half and four ATP molecules are formed during the 2nd half. This produces a internet gain of two ATP molecules per molecule of glucose for the prison cell.
Practice Question
Both prokaryotic and eukaryotic organisms carry out some form of glycolysis. How does that fact support or non support the assertion that glycolysis is one of the oldest metabolic pathways?
[reveal-respond q="619457″]Show Reply[/reveal-answer]
[subconscious-answer a="619457″]If glycolysis evolved relatively belatedly, it probable would not exist as universal in organisms equally it is. It probably evolved in very primitive organisms and persisted, with the improver of other pathways of carbohydrate metabolism that evolved later.[/hidden-answer]
Citric Acid Cycle and Oxidative Phosphorylation
The Citric Acid Cycle
In eukaryotic cells, the pyruvate molecules produced at the stop of glycolysis are transported into mitochondria, which are sites of cellular respiration. If oxygen is available, aerobic respiration will get forward. In mitochondria, pyruvate will exist transformed into a ii-carbon acetyl group (by removing a molecule of carbon dioxide) that will exist picked upwardly by a carrier chemical compound called coenzyme A (CoA), which is made from vitamin B5. The resulting compound is chosen acetyl CoA. (Effigy four). Acetyl CoA tin can be used in a variety of ways by the cell, but its major role is to deliver the acetyl group derived from pyruvate to the side by side pathway in glucose catabolism.

Similar the conversion of pyruvate to acetyl CoA, the citric acid cycle in eukaryotic cells takes place in the matrix of the mitochondria. Unlike glycolysis, the citric acid wheel is a closed loop: The concluding function of the pathway regenerates the chemical compound used in the first step. The 8 steps of the bike are a series of chemical reactions that produces two carbon dioxide molecules, one ATP molecule (or an equivalent), and reduced forms (NADH and FADH2) of NAD+ and FAD+, important coenzymes in the prison cell. Part of this is considered an aerobic pathway (oxygen-requiring) considering the NADH and FADHii produced must transfer their electrons to the adjacent pathway in the organization, which will use oxygen. If oxygen is not present, this transfer does non occur.
Ii carbon atoms come up into the citric acid cycle from each acetyl group. 2 carbon dioxide molecules are released on each turn of the bicycle; however, these exercise not contain the same carbon atoms contributed past the acetyl group on that plow of the pathway. The two acetyl-carbon atoms will eventually be released on later turns of the cycle; in this manner, all six carbon atoms from the original glucose molecule volition be somewhen released equally carbon dioxide. It takes two turns of the cycle to process the equivalent of one glucose molecule. Each plough of the bicycle forms three high-energy NADH molecules and one high-energy FADH2 molecule. These high-free energy carriers will connect with the last portion of aerobic respiration to produce ATP molecules. I ATP (or an equivalent) is likewise made in each cycle. Several of the intermediate compounds in the citric acrid cycle tin be used in synthesizing non-essential amino acids; therefore, the bicycle is both anabolic and catabolic.
Oxidative Phosphorylation
You take just read about 2 pathways in glucose catabolism—glycolysis and the citric acid bicycle—that generate ATP. Most of the ATP generated during the aerobic catabolism of glucose, however, is not generated direct from these pathways. Rather, it derives from a procedure that begins with passing electrons through a series of chemical reactions to a terminal electron acceptor, oxygen. These reactions have place in specialized poly peptide complexes located in the inner membrane of the mitochondria of eukaryotic organisms and on the inner part of the cell membrane of prokaryotic organisms. The free energy of the electrons is harvested and used to generate a electrochemical slope beyond the inner mitochondrial membrane. The potential energy of this gradient is used to generate ATP. The entirety of this process is called oxidative phosphorylation.
The electron ship concatenation (Figure 5a) is the last component of aerobic respiration and is the only role of metabolism that uses atmospheric oxygen. Oxygen continuously diffuses into plants for this purpose. In animals, oxygen enters the torso through the respiratory system. Electron transport is a serial of chemical reactions that resembles a bucket brigade in that electrons are passed speedily from one component to the next, to the endpoint of the chain where oxygen is the final electron acceptor and water is produced. At that place are four complexes composed of proteins, labeled I through 4 in Figure 5c, and the assemblage of these four complexes, together with associated mobile, accompaniment electron carriers, is called the electron transport chain. The electron ship chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes and in the plasma membrane of prokaryotes. In each transfer of an electron through the electron transport concatenation, the electron loses energy, but with some transfers, the energy is stored as potential energy past using information technology to pump hydrogen ions beyond the inner mitochondrial membrane into the intermembrane space, creating an electrochemical gradient.

Electrons from NADH and FADH2 are passed to poly peptide complexes in the electron transport concatenation. Every bit they are passed from one complex to another (there are a total of four), the electrons lose energy, and some of that free energy is used to pump hydrogen ions from the mitochondrial matrix into the intermembrane space. In the quaternary poly peptide circuitous, the electrons are accustomed by oxygen, the terminal acceptor. The oxygen with its extra electrons then combines with two hydrogen ions, further enhancing the electrochemical slope, to form water. If there were no oxygen present in the mitochondrion, the electrons could non be removed from the arrangement, and the entire electron transport concatenation would dorsum upwardly and stop. The mitochondria would exist unable to generate new ATP in this manner, and the cell would ultimately die from lack of energy. This is the reason we must breathe to draw in new oxygen.
In the electron send concatenation, the free free energy from the series of reactions only described is used to pump hydrogen ions across the membrane. The uneven distribution of H+ ions across the membrane establishes an electrochemical gradient, owing to the H+ ions' positive charge and their higher concentration on one side of the membrane.
Hydrogen ions lengthened through the inner membrane through an integral membrane protein called ATP synthase (Figure 5b). This circuitous protein acts equally a tiny generator, turned by the forcefulness of the hydrogen ions diffusing through it, down their electrochemical gradient from the intermembrane space, where there are many mutually repelling hydrogen ions to the matrix, where at that place are few. The turning of the parts of this molecular auto regenerate ATP from ADP. This menstruum of hydrogen ions beyond the membrane through ATP synthase is called chemiosmosis.
Chemiosmosis (Effigy 5c) is used to generate 90 percent of the ATP fabricated during aerobic glucose catabolism. The result of the reactions is the production of ATP from the energy of the electrons removed from hydrogen atoms. These atoms were originally part of a glucose molecule. At the cease of the electron ship system, the electrons are used to reduce an oxygen molecule to oxygen ions. The extra electrons on the oxygen ions attract hydrogen ions (protons) from the surrounding medium, and water is formed. The electron transport chain and the product of ATP through chemiosmosis are collectively chosen oxidative phosphorylation.
ATP Yield
The number of ATP molecules generated from the catabolism of glucose varies. For case, the number of hydrogen ions that the electron transport chain complexes can pump through the membrane varies between species. Another source of variance stems from the shuttle of electrons across the mitochondrial membrane. The NADH generated from glycolysis cannot easily enter mitochondria. Thus, electrons are picked up on the inside of the mitochondria by either NAD+ or FAD+. Fewer ATP molecules are generated when FAD+ acts as a carrier. NAD+ is used as the electron transporter in the liver and FAD+ in the brain, and so ATP yield depends on the tissue being considered.
Another cistron that affects the yield of ATP molecules generated from glucose is that intermediate compounds in these pathways are used for other purposes. Glucose catabolism connects with the pathways that build or intermission down all other biochemical compounds in cells, and the result is somewhat messier than the ideal situations described thus far. For case, sugars other than glucose are fed into the glycolytic pathway for energy extraction. Other molecules that would otherwise be used to harvest energy in glycolysis or the citric acrid bike may exist removed to form nucleic acids, amino acids, lipids, or other compounds. Overall, in living systems, these pathways of glucose catabolism extract about 34 percentage of the energy contained in glucose.
Learning Objectives
The citric acid cycle is a series of chemic reactions that removes loftier-free energy electrons and uses them in the electron transport chain to generate ATP. One molecule of ATP (or an equivalent) is produced per each turn of the cycle.
The electron transport chain is the portion of aerobic respiration that uses free oxygen equally the concluding electron acceptor for electrons removed from the intermediate compounds in glucose catabolism. The electrons are passed through a series of chemic reactions, with a small amount of free energy used at three points to transport hydrogen ions across the membrane. This contributes to the gradient used in chemiosmosis. Equally the electrons are passed from NADH or FADH2 down the electron ship chain, they lose energy. The products of the electron transport chain are h2o and ATP. A number of intermediate compounds tin be diverted into the anabolism of other biochemical molecules, such every bit nucleic acids, non-essential amino acids, sugars, and lipids. These aforementioned molecules, except nucleic acids, can serve equally energy sources for the glucose pathway.
Practice Questions
Cyanide inhibits cytochrome c oxidase, a component of the electron transport concatenation. If cyanide poisoning occurs, would you lot expect the pH of the intermembrane space to increase or subtract? What affect would cyanide accept on ATP synthesis?
[practice-expanse rows="4″][/practice-area]
[reveal-answer q="451713″]Prove Answer[/reveal-reply]
[hidden-answer a="451713″]Afterward cyanide poisoning, the electron transport chain can no longer pump electrons into the intermembrane space. The pH of the intermembrane space would increase, and ATP synthesis would stop.
[/hidden-answer]
We inhale oxygen when we breathe and exhale carbon dioxide. What is the oxygen used for and where does the carbon dioxide come from?
[practice-area rows="4″][/practice-expanse]
[reveal-answer q="985212″]Show Answer[/reveal-reply]
[hidden-answer a="985212″]The oxygen nosotros inhale is the final electron acceptor in the electron ship chain and allows aerobic respiration to keep, which is the most efficient pathway for harvesting free energy in the form of ATP from nutrient molecules. The carbon dioxide we exhale out is formed during the citric acid wheel when the bonds in carbon compounds are broken.[/hidden-answer]
Summary
Cellular respiration is a process that all living things use to convert glucose into energy. Autotrophs (like plants) produce glucose during photosynthesis. Heterotrophs (similar humans) ingest other living things to obtain glucose. While the procedure tin seem complex, this page takes you through the central elements of each part of cellular respiration.
Allow's Review
Cellular respiration is a drove of three unique metabolic pathways: glycolysis, the citric acrid cycle, and the electron transport chain. Glycolysis is an anaerobic process, while the other two pathways are aerobic. In club to move from glycolysis to the citric acid cycle, pyruvate molecules (the output of glycolysis) must be oxidized in a process chosen pyruvate oxidation.
Glycolysis
Glycolysis is the first pathway in cellular respiration. This pathway is anaerobic and takes place in the cytoplasm of the cell. This pathway breaks downwardly 1 glucose molecule and produces 2 pyruvate molecules. At that place are ii halves of glycolysis, with five steps in each half. The first half is known as the "free energy requiring" steps. This half splits glucose, and uses upwardly 2 ATP. If the concentration of pyruvate kinase is high enough, the second half of glycolysis can proceed. In the 2nd half, the "free energy releasing: steps, 4 molecules of ATP and 2 NADH are released. Glycolysis has a net gain of 2 ATP molecules and 2 NADH.
Some cells (due east.g., mature mammalian red blood cells) cannot undergo aerobic respiration, and so glycolysis is their just source of ATP. However, nigh cells undergo pyruvate oxidation and go along to the other pathways of cellular respiration.
Pyruvate Oxidation
In eukaryotes, pyruvate oxidation takes place in the mitochondria. Pyruvate oxidation tin simply happen if oxygen is bachelor. In this procedure, the pyruvate created by glycolysis is oxidized. In this oxidation process, a carboxyl grouping is removed from pyruvate, creating acetyl groups, which compound with coenzyme A (CoA) to form acetyl CoA. This process as well releases CO2.
Citric Acid Cycle
The citric acrid cycle (also known as the Krebs cycle) is the second pathway in cellular respiration, and information technology besides takes identify in the mitochondria. The rate of the cycle is controlled by ATP concentration. When there is more ATP available, the rate slows downwardly; when there is less ATP the rate increases. This pathway is a closed loop: the final step produces the compound needed for the outset stride.
The citric acrid bike is considered an aerobic pathway because the NADH and FADH2 it produces act every bit temporary electron storage compounds, transferring their electrons to the next pathway (electron send chain), which uses atmospheric oxygen. Each plough of the citric acid cycle provides a net gain of CO2, 1 GTP or ATP, and three NADH and 1 FADH2.
Electron Send Chain
About ATP from glucose is generated in the electron transport chain. It is the just part of cellular respiration that directly consumes oxygen; however, in some prokaryotes, this is an anaerobic pathway. In eukaryotes, this pathway takes identify in the inner mitochondrial membrane. In prokaryotes it occurs in the plasma membrane.
The electron transport chain is made upwards of four proteins forth the membrane and a proton pump. A cofactor shuttles electrons between proteins I–III. If NAD is depleted, skip I: FADH2 starts on II. In chemiosmosis, a proton pump takes hydrogens from inside mitochondria to the exterior; this spins the "motor" and the phosphate groups adhere to that. The movement changes from ADP to ATP, creating 90% of ATP obtained from aerobic glucose catabolism.
Let's Practice
Now that you've reviewed cellular respiration, this practice activity will help you see how well you know cellular respiration:
A link to an interactive elements can be found at the bottom of this page.
Click here for a text-merely version of the activity.
Check Your Understanding
Answer the question(south) below to see how well you understand the topics covered in the previous section. This short quiz does not count toward your grade in the class, and y'all can retake it an unlimited number of times.
Utilise this quiz to check your understanding and decide whether to (1) study the previous section farther or (two) movement on to the next section.
https://assessments.lumenlearning.co...sessments/6878
gaillardwharroposs.blogspot.com
Source: https://bio.libretexts.org/Courses/Lumen_Learning/Book%3A_Biology_for_Non-Majors_I_(Lumen)/06%3A_Metabolic_Pathways/6.04%3A_Cellular_Respiration
0 Response to "Is Cellular Respiration an Anabolic or Catabolic Pathway? How Do You Know?"
Post a Comment